Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ichihara, Akira; Matsuoka, Leo*
no journal, ,
Separation of long-lived fission products from other isotopes is important for the research of nuclear transmutation. Recently, we proposed an isotope separation method applicable to diatomic molecules in the gas phase. In this method, two kinds of terahertz (THz) optical fields are utilized to dissociate the selected isotope molecule. In this study, we investigated the isotope-selective dissociation using the 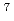 Li
Li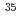 Cl and
Cl and 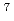 Li
Li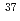 Cl molecules in the rotational temperature of 70 K, where LiCl was employed as a substitute of CsI which is formed in nuclear reactors. The molecular wave-packet (WP) computation was carried out to demonstrate that the
Cl molecules in the rotational temperature of 70 K, where LiCl was employed as a substitute of CsI which is formed in nuclear reactors. The molecular wave-packet (WP) computation was carried out to demonstrate that the 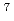 Li
Li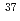 Cl molecules can be dissociated selectively by using the THz optical pulses. The obtained dissociation probabilities were 20 % for
Cl molecules can be dissociated selectively by using the THz optical pulses. The obtained dissociation probabilities were 20 % for 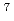 Li
Li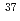 Cl, and 0.5 % for
Cl, and 0.5 % for 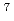 Li
Li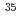 Cl, respectively. We expect that this method is applicable to other diatomic molecular ensembles whose rotational states are in the thermal distribution.
Cl, respectively. We expect that this method is applicable to other diatomic molecular ensembles whose rotational states are in the thermal distribution.
Kaneko, Masashi
no journal, ,
Density functional theory (DFT) calculations have been employed to understand the equilibrium structures, electronic states, and stabilities of minor-actinides (MA) and lanthanides (Ln) complexes. An previous application of DFT calculation to the MA/Ln separation has indicated that the Am/Eu selectivity can be explained by the stability in complexation reaction. However, the origin of Am/Eu selectivity remains unclear. This study aims to understand the origin by correlating the stability of their complexes with the covalency in metal-ligand coordination bonds by means of scalar-relativistic DFT calculations. After an optimization of DFT method using benchmark study with M ssbauer spectroscopic parameters, we applied the DFT method to the separation of Am from Eu and analyzed the bonding states between metal and ligands in Eu and Am complexes. As a result, it was found that the wave functions of Eu ion with ligands have syn-phase overlap not depending on donor atoms, whereas those of Am ion have syn-phase overlap in the case of sulfur donors and anti-phase in the case of oxygen donors. This indicates that the difference in bonding states between metal and ligands is an origin in the Am/Eu selectivity.
ssbauer spectroscopic parameters, we applied the DFT method to the separation of Am from Eu and analyzed the bonding states between metal and ligands in Eu and Am complexes. As a result, it was found that the wave functions of Eu ion with ligands have syn-phase overlap not depending on donor atoms, whereas those of Am ion have syn-phase overlap in the case of sulfur donors and anti-phase in the case of oxygen donors. This indicates that the difference in bonding states between metal and ligands is an origin in the Am/Eu selectivity.
Murayama, Tatsuya*; Watanabe, Masayuki; Aoyagi, Noboru; Fujisawa, Kiyoshi*
no journal, ,
In this study, we synthesized Eu(III) complex using N,N,N',N'-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN) and TPEN derivatives, N,N,N',N'-tetrakis(2-pyridylmethyl)-propylenediamine(MeTPEN) which has methyl group into the ethylenediamine framework, the structure and physical properties of these complexes investigated. The crystal structure of Eu(TPEN) and Eu(MeTPEN), the geometry of these structures indicate pseudo-bicapped square antiprism (10 coordination) with two bidentate NO
 ; ions. As the result of investigation of fluorescence spectrum and lifetime in organic solution, the fluorescence of Eu (MeTPEN) was enhanced more intensity than that of Eu(TPEN) by addition of OAc
; ions. As the result of investigation of fluorescence spectrum and lifetime in organic solution, the fluorescence of Eu (MeTPEN) was enhanced more intensity than that of Eu(TPEN) by addition of OAc ; ion.
; ion.
Sato, Tetsuya; Kaneya, Yusuke*; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko; Makii, Hiroyuki; Hirose, Kentaro; Nagame, Yuichiro; et al.
no journal, ,
Our experimental results on the first ionization potential measurement of lawrencium (Lr, element 103) have strongly suggested that the Lr atom has a [Rn]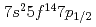 configuration as a result of the influence of strong relativistic effects. The configuration is different from that expected from the lanthanide homologue, lutetium (Lu). According to a semi-empirical consideration, it is expected that the change of the electronic configuration leads higher volatility of Lr than that of Lu. In this work, adsorption behaviors of Lr and various short-lived rare earth isotopes on a tantalum surface were investigated via observation of their surface ionization efficiencies. It was found that Lr would behave like low volatile rare earth elements such as Lu contrary to the semi-empirical expectation.
configuration as a result of the influence of strong relativistic effects. The configuration is different from that expected from the lanthanide homologue, lutetium (Lu). According to a semi-empirical consideration, it is expected that the change of the electronic configuration leads higher volatility of Lr than that of Lu. In this work, adsorption behaviors of Lr and various short-lived rare earth isotopes on a tantalum surface were investigated via observation of their surface ionization efficiencies. It was found that Lr would behave like low volatile rare earth elements such as Lu contrary to the semi-empirical expectation.
Shimojo, Kojiro; Yabe, Makoto*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
Solvent extraction is an effective method for the separation and purification of metal ions. The extractant plays a key role in the extraction efficiency and the separation operation. In this study, we have synthesized calix[4]arene diglycolamic acid derivative as a novel extractant and have investigated extraction behavior of lanthanide ions. It was found that the pre-organization of four diglycolamic acid groups in the cyclic structure can improve remarkably extraction performance and separation ability for lanthanide ions.
Ouchi, Kazuki; Otobe, Haruyoshi; Kitatsuji, Yoshihiro
no journal, ,
We investigated the relationship between the quantity of electricity of electrolytic reduction of U(VI) to U(IV) and the deposition on the electrode surface by measuring electrochemical quartz crystal microbalance of U(IV) deposits following the electrolytic reduction of U(VI) to U(V) in a pH4 solution. We found that the deposition can be divided into the two phases after starting deposition according to variation between electric quantity and deposition amount. This variation indicates that the species of deposits in first and second phase are highly likely to be U(IV) hydroxide (U(OH) ) and UO
) and UO , respectively. From this result, we propose the deposition mechanism that a U hydroxide forms as an intermediate and transforms to U oxide as a terminal product.
, respectively. From this result, we propose the deposition mechanism that a U hydroxide forms as an intermediate and transforms to U oxide as a terminal product.